A spokesperson for the company responded to CBS News, however, saying that Infinant Health is planning to continue distributing its “Evivo powder product” for consumers to buy and intends “to work with the FDA toward approval of the use of our MCT oil product in hospital settings.”


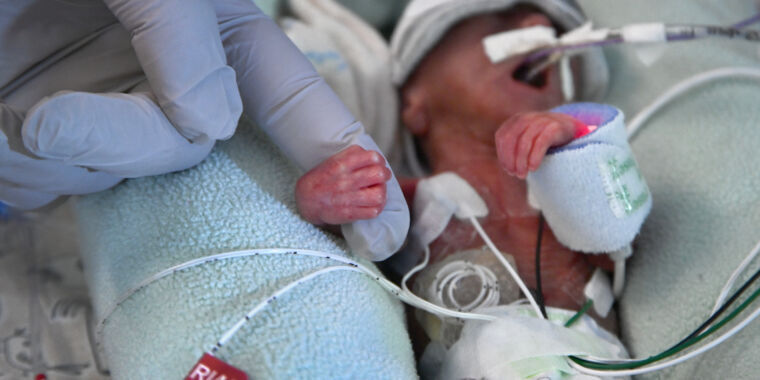
Why the FDA is upset specifically: “Moreover, the agency took aim at the company touting the supplement as “made specifically for use in healthcare settings,” and “[d]esigned specifically for infants in the NICU[.]” NICU is the Neonatal Intensive Care Unit, where the most vulnerable of newborns, many preterm, receive specialized and intensive care.”
And also they claim their study design was bad and inadequate to proof efficacy.
But the article also says 10% of such cases recieve such probiotic products. Would be interesting to see what the outcomes of those 10% are vs the 90%.